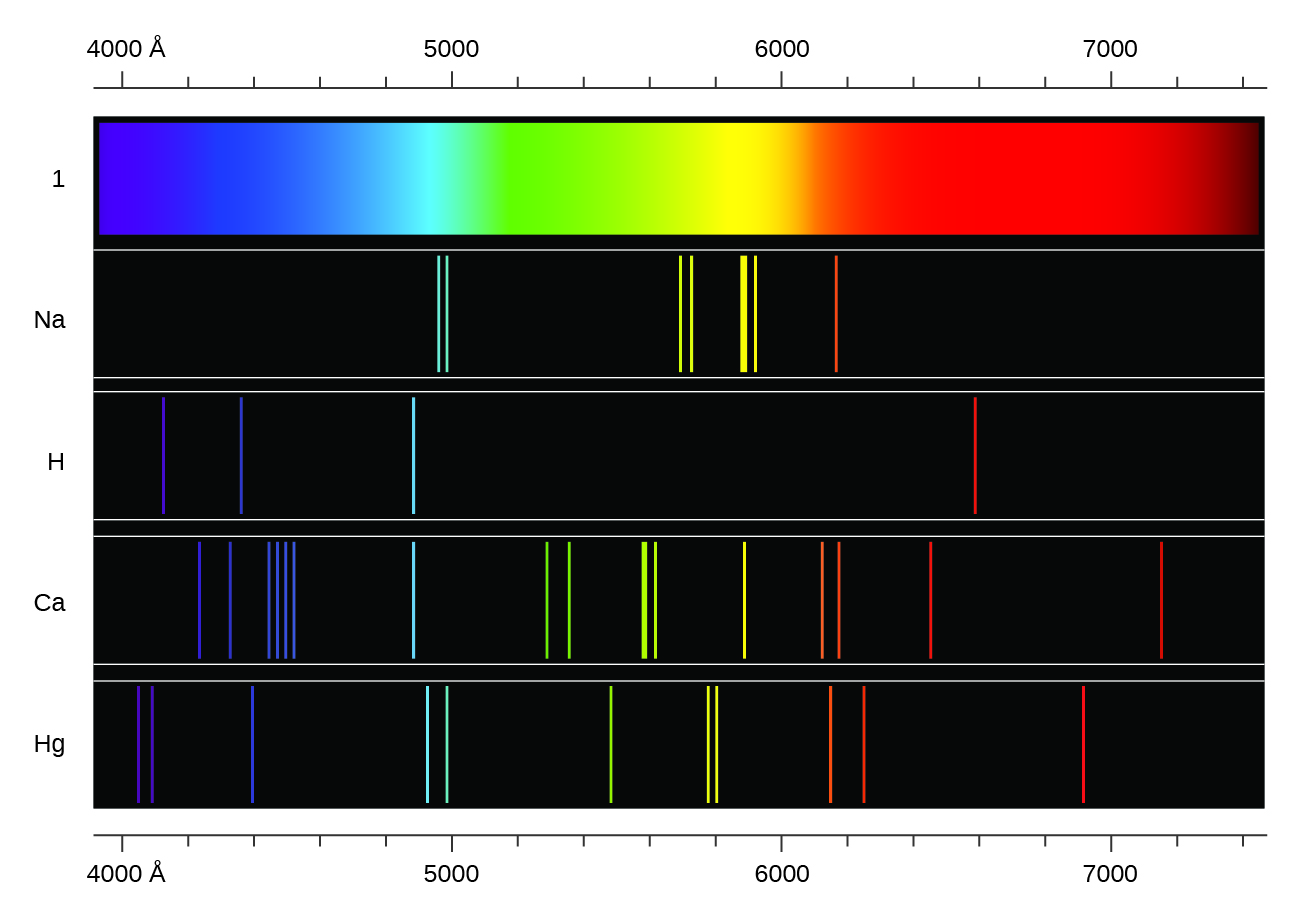
Lines Spectra and Excited Electron States

Atomic Spectra and Applications, by Ibrar Ahmad
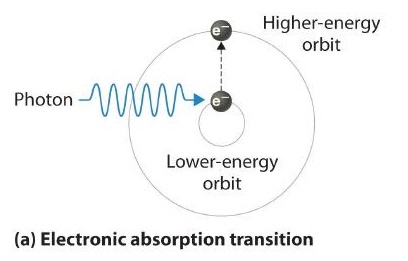
6.3: Line Spectra and the Bohr Model - Chemistry LibreTexts

Atomic Spectra
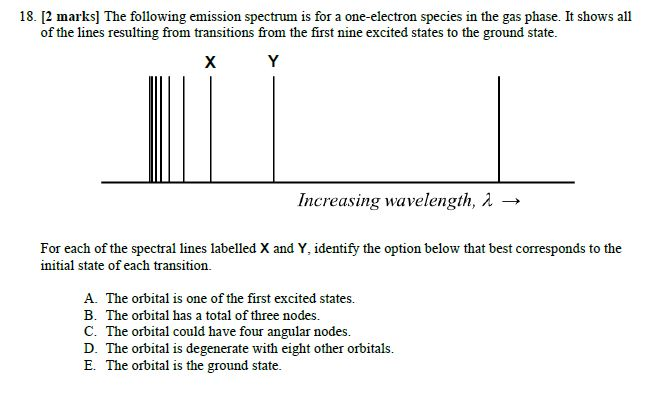
Solved 18. [2 marks] The following emission spectrum is for

2.2 Hydrogen emission spectrum (SL)

34. ☆ A certain electronic transition from an excited state to ground state of the H, atom in one or step gives rise to three lines in the ultra violet region of

1 2 3 4 5 6 A certain electronic transition from an excited state to ground state of the H, atom in one or step gives rise to three lines in the
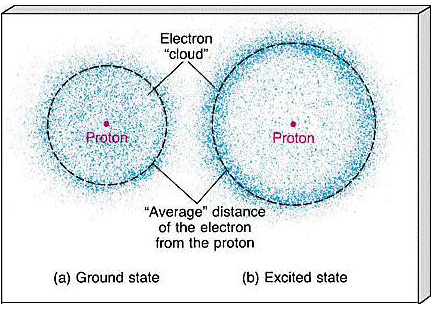
Chapter 4, Section 2
A certain transition in H spectrum from an exited state to ground state in one or steps gives rise to total 10 lines. How many of these belong to paschen series?
What causes spectral lines? - Quora
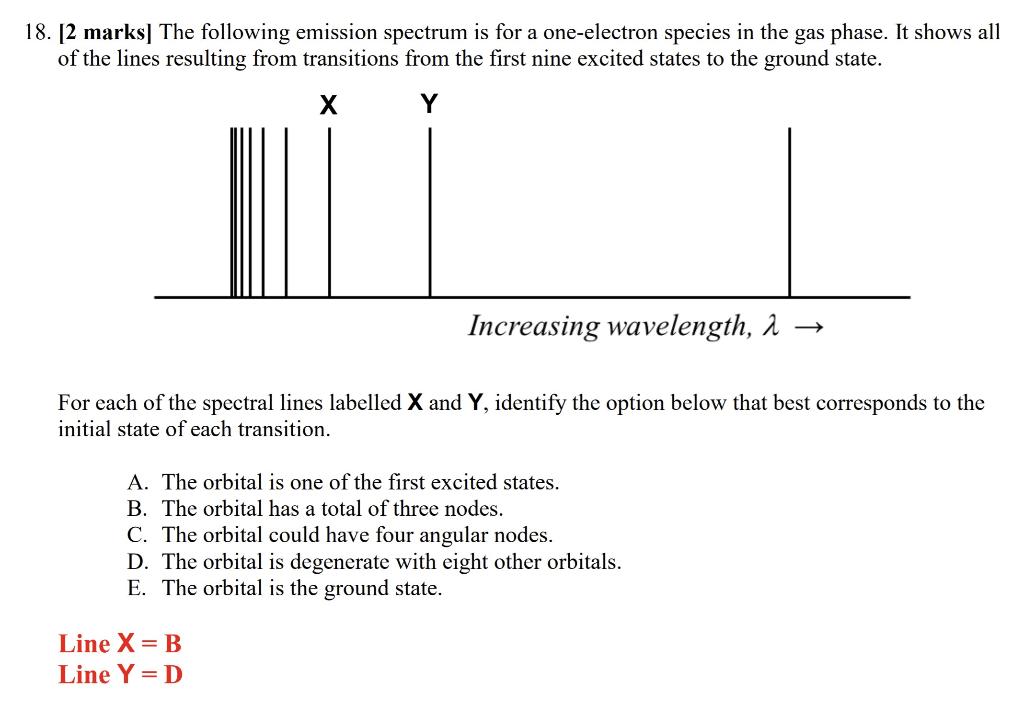
Solved 18. [2 marks] The following emission spectrum is for
What is emission spectrum? - Quora
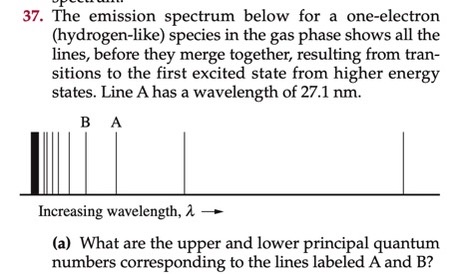
SOLVED: 37. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the first excited state from
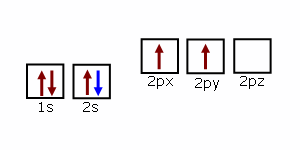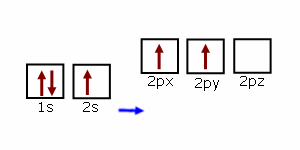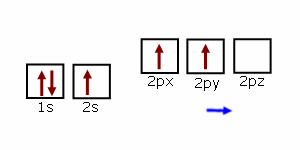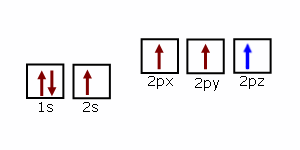
Lines Spectra and Excited Electron States
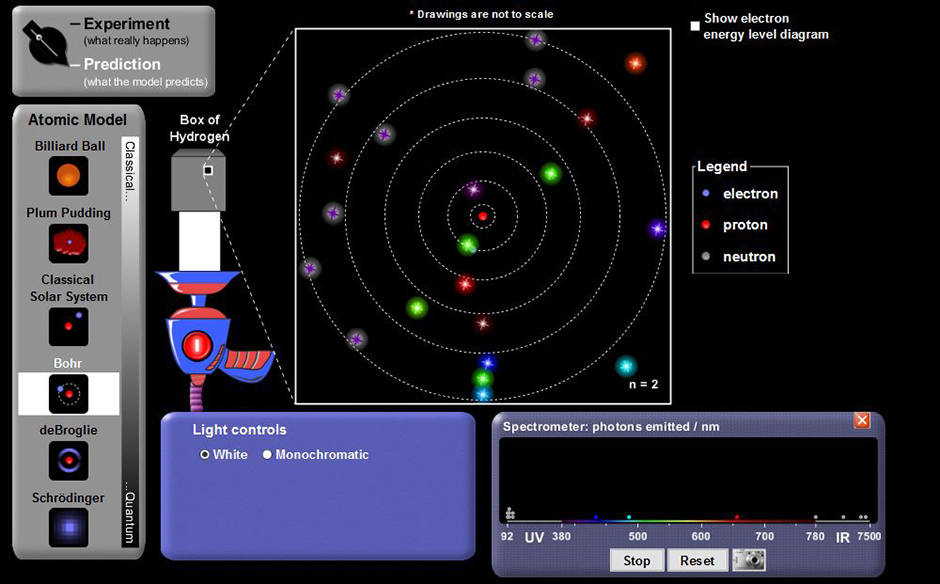
hydrogen spectrum






